| Safety Data Sheet - Heparinase I |
| SECTION 1 – IDENTIFICATION | |
| Release Date | May 2019 R. 01 |
| Product Identity | Heparinase I, recombinant in sucrose, Research Grade |
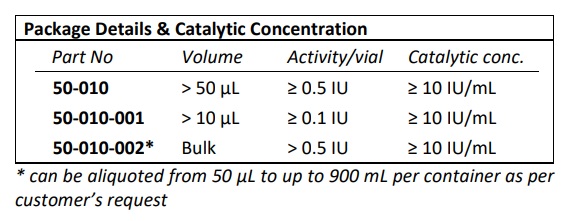
| Part Number | 50-010 |
| Manufacturer | IBEX Pharmaceuticals Inc.
5485 Rue Paré, Suite 100
Montréal, Québec, H4P 1P7 Canada
Tel : (514) 344-4004 Fax : (514) 344-8827 |
| Product use | Research |
| |
| SECTION 2 – HAZARD IDENTIFICATION | |
| Emergency overview | Solution in water. Will not support burning. Not explosive. |
| Potential Health Effects | |
| Skin | May cause skin irritation |
| Eyes | May cause irritation or burning sensation |
| Inhalation | May cause irritation to the respiratory tract |
| Swallowed | Not known to cause toxic effects |
| Injection | Not known to cause toxicity with acute exposure, but may produce toxicity with repeated injections. |
| Carcinogenicity | Not determined |
| SECTION 3 – COMPOSITION/INFORMATION ON INGREDIENTS | |
| CAS No. | 9025-39-2 |
| EC No. | 4.2.2.7 |
| Systematic name | Heparin lyase |
| Synonyms | Heparin eliminase
Heparinase I |
| Origin | Recombinant, non-pathogenic microorganism |
| Ingredients | No components need to be disclosed according to the applicable regulations. |
| SECTION 4 – FIRST AID MEASURES | |
| Skin contact | Flush with copious amounts of water. |
| Eye contact | Rinse opened eye for several minutes under running water. Then consult a doctor. |
| Inhalation | Supply the victim with fresh air; consult doctor in case of complaints. |
| Swallowing | Rinse mouth with plenty of water. |
| On all of the above | Consult a doctor if symptoms develop. |
| SECTION 5 – FIRE FIGHTING MEASURES | |
| Suitable extinguishing agents | Noncombustible. |
| Protective equipment | No special measures required. Prevent contact with skin and eyes |
| SECTION 6 – ACCIDENTAL RELEASE MEASURES | |
| Person-related safety precautions | Prevent contact with skin and eyes. Ventilate area. |
| Measures for environmental protection | No special measures required. |
| Measures for cleaning/collecting | Spilled material should be carefully wiped up and disposed as biomedical waste as per applicable waste disposal regulations. Wash spill site after material pickup is complete. |
| SECTION 7 – HANDLING AND STORAGE | |
| Handling | |
| Information for safe handling | Prevent contact with skin and eyes and prevent inhalation and swallowing. No other special precautions are necessary if used correctly. |
| Information about protection against explosions and fires | No special measures required. |
| Storage | |
| Requirements to be met by storerooms and receptacles | No special requirements except that product should be stored frozen (preferably at -70 °C). |
| Information about storage in one common storage facilit | Not required. |
| Further information about storage conditions | None |
| Storage class | None assigned |
| Class according to regulation on flammable liquids | Void |
| SECTION 8 – EXPOSURE CONTROLS / PERSONAL PROTECTION | |
| General protective and hygienic measures | The usual precautionary measures for handling chemicals and potentially biohazardous materials should be followed. Use clothing sufficient to avoid skin contact. Safety shower and eye wash should be available in proximity. |
| Protection of hands | Use synthetic water resistant gloves. |
| Eye protection | Use safety glasses. |
| SECTION 9 – PHYSICAL AND CHEMICAL PROPERTIES | |
| Physical form | Frozen, colorless, odorless aqueous solution.
(Heparinase I is a protein) |
| Melting point | 0 °C (melting point of aqueous solution) |
| Boiling point | Not applicable |
| Flash point | Not applicable |
| Flammability | Product is not flammable |
| Danger of explosion | Not determined |
| Solubility | Heparinase I and ingredients are water soluble |
| Organic solvents | 0 % |
| Solids content | 0 % |
| SECTION 10 – STABILITY AND REACTIVITY | |
| Thermal decomposition / conditions to be avoided | No decomposition if used according to specifications. |
| Dangerous reactions | No dangerous reactions known. |
| Dangerous products of decomposition | No dangerous decomposition products known. |
| SECTION 11 – TOXICOLOGICAL INFORMATION | |
| Effects of Acute Exposure | Low acute toxicity expected following inhalation, ingestion, injection or skin exposure. The potential eye irritation effects of Heparinase I are unknown and it should be considered to be potentially irritating to the eyes. |
| Effects of Chronic Exposure | Not determined for oral ingestion or skin exposure |
| Development Toxicity | Not determined |
| Reproductive Toxicity | Not determined |
| Carcinogenicity | Not determined |
| SECTION 12 – ECOLOGICAL INFORMATION | |
| Toxicity | No data available |
| Persistence and degradability | No data available |
| Bioaccumulative potential | No data available |
| Mobility in soil | No data available |
| PBT and vPvB assessment | No data available |
| Other adverse effects | No data available |
| SECTION 13 – DISPOSAL INFORMATION | |
| Waste Disposal | Incinerate or bury as a solid in a licensed facility. Do not discharge into waterways or sewer systems without proper authority. |
| Container Disposal | Dispose of in a licensed facility. Recommend crushing or other means to prevent unauthorized reuse. |
| SECTION 14 – TRANSPORT INFORMATION | |
| DOT (US) | Not dangerous goods |
| IMDG | Not dangerous goods |
| IATA | Not dangerous goods |
| SECTION 15 – REGULATORY INFORMATION | |
| Not a dangerous substance or mixture according to the Globally Harmonised System (GHS). | |
| SECTION 16 – OTHER INFORMATION | |
| This information is given without any warranty or representation. It is believed to be correct but does not claim to be all inclusive and shall be used only as a guide. IBEX Pharmaceuticals Inc. shall not be held liable for any damage resulting from handling or contact with the above product. It is offered solely for your consideration, investigation and verification. | |
| |
| |
| |
| |
| |
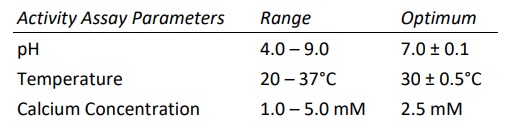
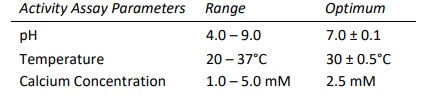
 Heparinase I, also known as Heparin Lyase I, is endolytically and exolytically active on both heparin and highly sulfated domains of heparan sulfate. It cleaves selectively, via an elimination mechanism, highly sulfated polysaccharide chains containing 1-4 linkages between hexosamines and o-sulfated iduronic acid residues. The reaction yields oligosaccharide products (mainly disaccharides) containing unsaturated uronic acids which can be detected by UV spectroscopy at 232 nm. The enzyme also cleaves the antithrombin III binding pentasaccharide domain in the heparin molecule.
Heparinase I, also known as Heparin Lyase I, is endolytically and exolytically active on both heparin and highly sulfated domains of heparan sulfate. It cleaves selectively, via an elimination mechanism, highly sulfated polysaccharide chains containing 1-4 linkages between hexosamines and o-sulfated iduronic acid residues. The reaction yields oligosaccharide products (mainly disaccharides) containing unsaturated uronic acids which can be detected by UV spectroscopy at 232 nm. The enzyme also cleaves the antithrombin III binding pentasaccharide domain in the heparin molecule.