Heparinases.
Using high purity enzymes will provide Reliable and Reproducible results
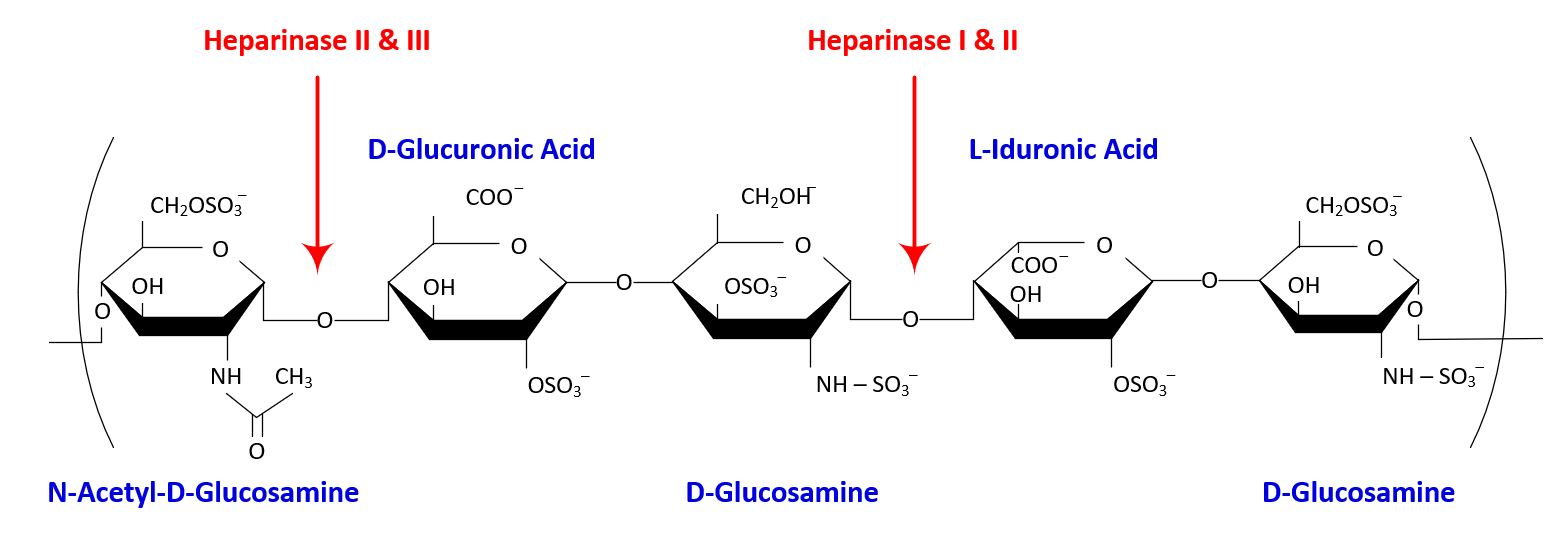
The catalytic targets of heparinize I, II and III are shown in the figure below
IBEX has developed a proprietary Pedobacter heparinus (formerly Flavobacterium heparinum) expression system which unlike other heparinase expression systems produces glycosylated GAG enzymes (with the exception of Heparinase III).
Enzymes purified from the native strain of P. heparinus (formerly F. heparinum) will almost certainly contain other GAG enzymes as contaminants.
Due to its high purity and reaction time, IBEX Heparinase I is the only heparinase used in FDA and EU approved hemostasis-measuring Point Of Care medical diagnostic devices.
IBEX heparinase I is also used in conjunction with many devices which monitor the anticoagulant levels in the patient blood such as the viscoelastic hemostatic Thromboelastography (TEG) devices, INR monitoring devices and many more.
IBEX heparinases are also widely used in the production of low and ultra-low molecular weight heparins.
The IBEX Pharmaceutical’s Quality Management System is certified ISO 13485:2016 for the manufacture and sale of protein reagents and reagent-filled medical diagnostic device components.
About Glycosaminoglycans (GAGs)
IBEX produces a family of high purity recombinant glycosaminoglycan (GAG) lyases; among them are Heparinase I, Heparinase II, Heparinase III, which are widely used in research, the production of low molecular weight heparin and ultra-low molecular weight heparins, and in conjunction with clinical diagnostic IVDs for hemostasis monitoring.
References
- Fritz, T. et al. (1994) Biol. Chem.269, 28809-28814. PMID: 7759502
- Linhardt, R.J. et al. (1990) Biochemistry29, 2611-2617. PMID: 2334685
- Linhardt, R.J., and Gunay, N.S. (1999) Thromb. Hemost. 25 Suppl 3, 5-16. PMID: 10549711
- Casu, B. et al. (2002) Biochemistry41, 10519-10528. PMID: 15106730
- Knudsen, C.B. and Knudsen, W. (2001) Cell Dev. Biol.12, 69-78. PMID: 11292372

Glycosaminoglycans (GAGs) are large linear-unbranched polysaccharide chains with repeating disaccharide units of amino sugar (either GlcNAc or GalNAc) and an uronic acid (either glucuronic acid and/or iduronic acid). Five glycosaminoglycan chains were identified, viz., Hyaluronan, Chondroitin, Dermatan, Heparin/heparan and Keratan with possible sulfation at 2S, 4S or 6S locations.
In virtually all animal tissues, the cell membranes and the extracellular matrices (ECM) are decorated with glycosaminoglycans (GAGs) such as heparin and heparan sulfate (HS), which are linear sulfated polysaccharides. These GAGs play an intricate role in the ECM, as polyelectrolytes by specifically interacting with growth factors and other transient components of the ECM.
Heparin and HS have been associated with cell-biological processes, cell adhesion and regulation of enzymatic catalysis while HS chains have been shown to interact with a variety of growth factors (fibroblast growth factors and vascular endothelial growth factor), chemokines, ECM proteins, enzymes, and antithrombin. Additionally, heparin and HS molecules were found to mediate the viral adhesion and the infection process and considered as important factors in the host-pathogen interactions.
Heparin, low molecular weight heparins (LMWH) and ultra-low molecular weight heparins (ULMWH) are widely used as anticoagulant drugs and they have been shown to regulate cellular process by binding, stabilizing and activating various growth factors. Heparinases are used in the production of LMWH and ULMWH.





