Heparinase III
 Heparinase III, also known as Heparin Lyase III or heparan sulfate lyase/eliminase or Heparitinase I is an endolytic enzyme. It cleaves selectively and exclusively the heparan sulfate via an elimination mechanism, undersulfated polysaccharide chains or acetylated or unsubstituted 1-4 linkages between hexosamines and glucuronic acid residues. The reaction yields oligosaccharide products (mainly disaccharides) containing unsaturated uronic acids which can be detected by UV spectroscopy at 232 nm. The enzyme is active only on heparan sulfate and does not cleave heparin.
Heparinase III, also known as Heparin Lyase III or heparan sulfate lyase/eliminase or Heparitinase I is an endolytic enzyme. It cleaves selectively and exclusively the heparan sulfate via an elimination mechanism, undersulfated polysaccharide chains or acetylated or unsubstituted 1-4 linkages between hexosamines and glucuronic acid residues. The reaction yields oligosaccharide products (mainly disaccharides) containing unsaturated uronic acids which can be detected by UV spectroscopy at 232 nm. The enzyme is active only on heparan sulfate and does not cleave heparin.
Recombinant enzyme homologous to its native counterpart and expressed in the natural host, Pedobacter heparinus (formerly, Flavobacterium heparinum).
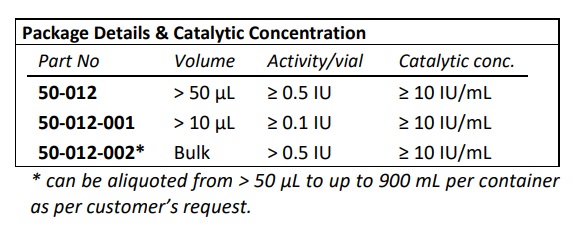
- EC Number: 4.2.2.8
- Purity: ≥ 95 % by reversed phase HPLC analysis
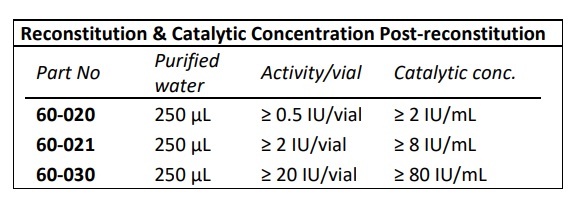
- IBEX liquid heparinase III has optimal activity and stability for up to 30 months when stored frozen at -70°C
- IBEX lyophilized heparinase I has optimal activity and stability for up to two years when stored at 2-8oC.
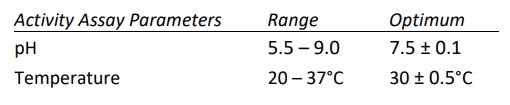
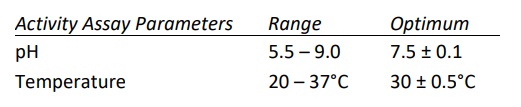
- Calcium dependent enzyme and has optimal activity in the presence of 1.5-5.0 mM CaCl2 . Highest activity observed when Ca+2 was at 10 mM
To request a quote, click the button below:

- Data Sheet - LIQUID
- Safety Data Sheet - LIQUID
- Data Sheet - LYOPHILIZED
- Safety Data Sheet - Lyophilized
Research Papers using IBEX Heparinase III
IBEX heparinase III (50-012-001) used to elucidate the mechanotransduction pathways for the shear-induced nitric oxide production and to improve the understanding of the cardiovascular disease progression. This will enable the design of future therapeutics to rescue endothelial dysfunction.
Heparan sulfate proteoglycan glypican‑1 and PECAM‑1 cooperate in shear‑induced endothelial nitric oxide production.
Abstract
This study aimed to clarify the role of glypican-1 and PECAM-1 in shear-induced nitric oxide production in endothelial cells. Atomic force microscopy pulling was used to apply force to glypican-1 and PECAM-1 on the surface of human umbilical vein endothelial cells and nitric oxide was measured using a fuorescent reporter dye. Glypican-1 pulling for 30 min stimulated nitric oxide production while PECAM-1 pulling did not. However, PECAM-1 downstream activation was necessary for the glypican-1 force-induced response. Glypican-1 knockout mice exhibited impaired fow-induced phosphorylation of eNOS without changes to PECAM-1 expression. A cooperation mechanism for the mechanotransduction of fuid shear stress to nitric oxide production was elucidated in which glypican-1 senses fow and phosphorylates PECAM-1 leading to endothelial nitric oxide synthase phosphorylation and nitric oxide production
 Click the scientific reports logo above to read the full paper.
Click the scientific reports logo above to read the full paper.
 Click the scientific reports logo above to read the full paper.
Click the scientific reports logo above to read the full paper.